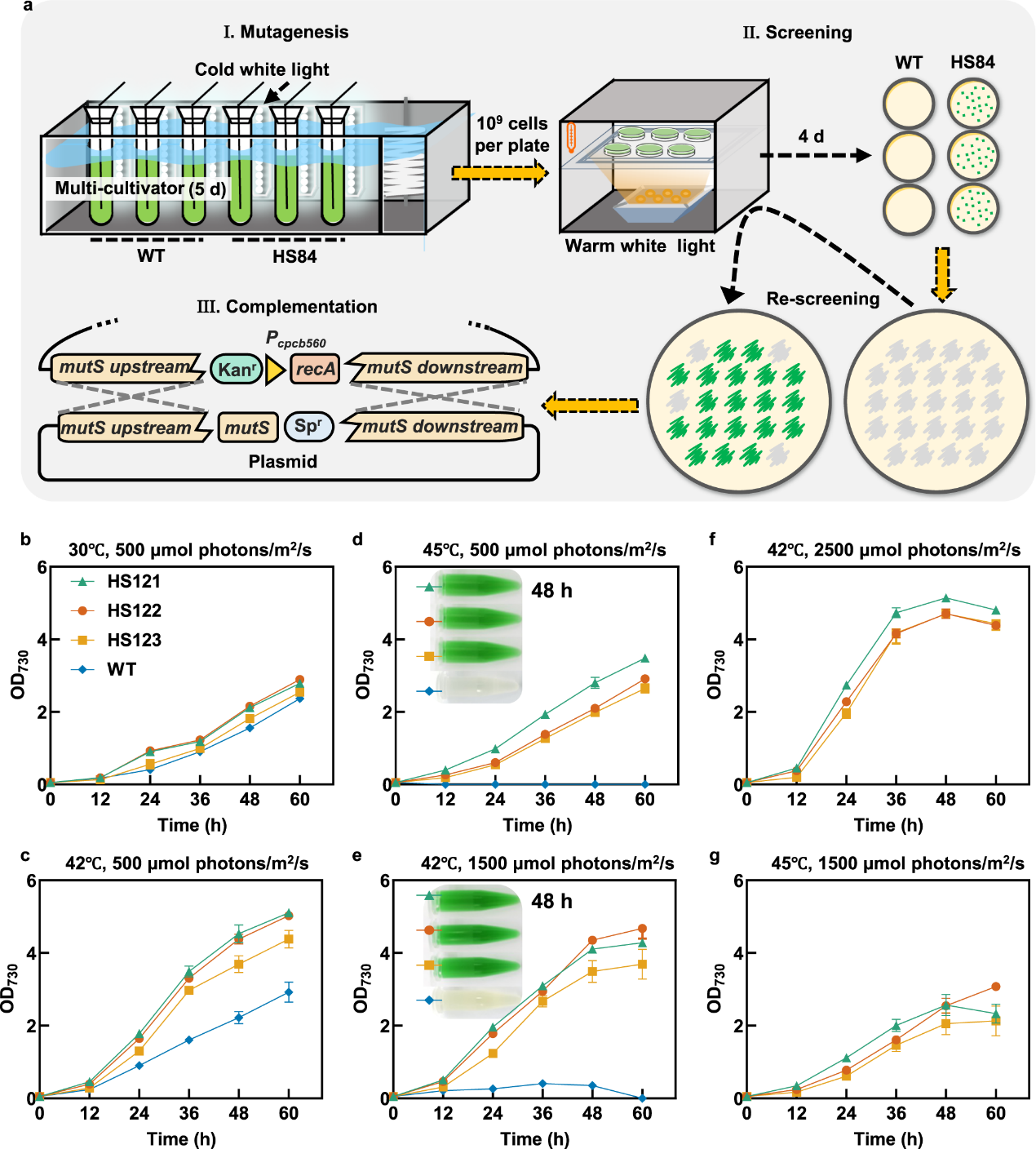
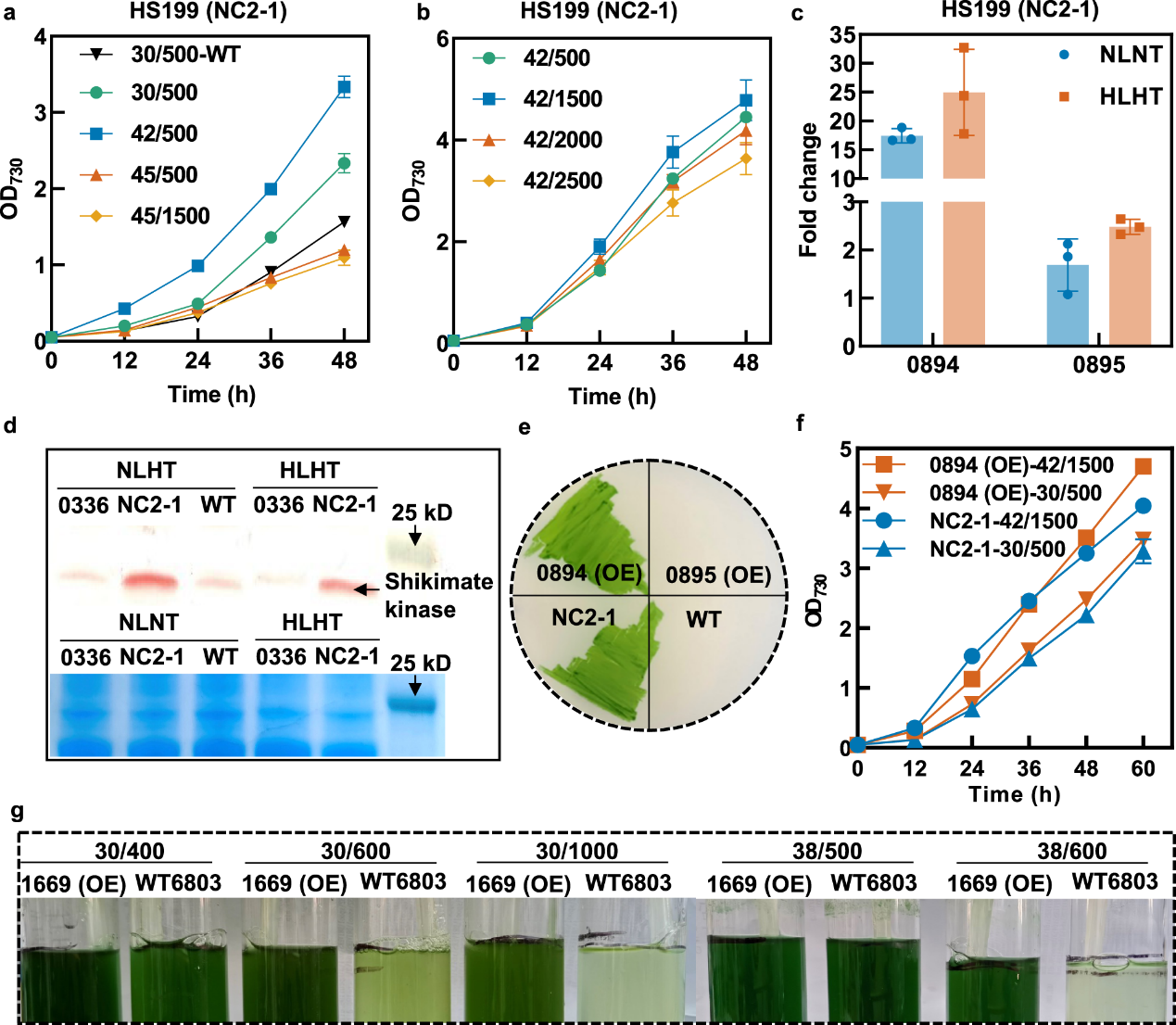
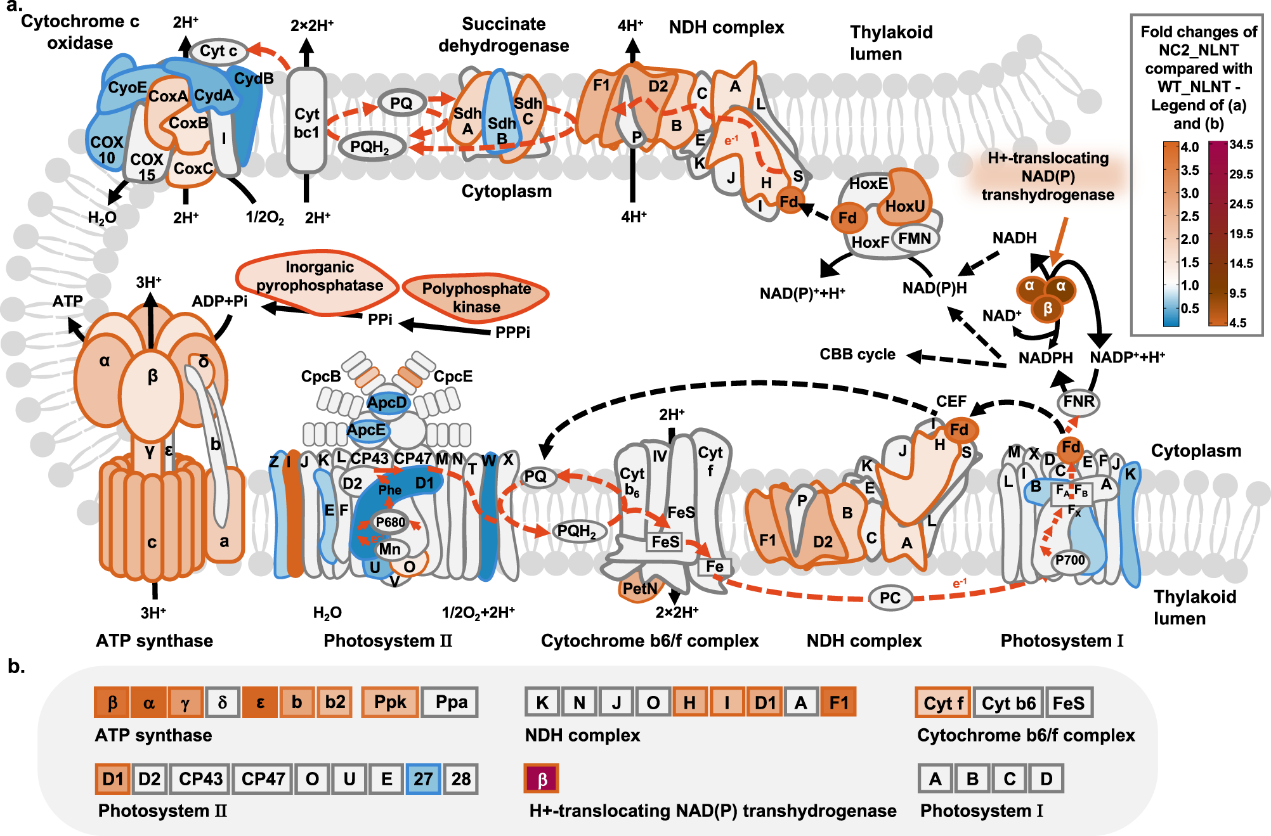
Photosynthesis is the most important biochemical process on Earth; through this process, photoautotrophs convert solar energy and carbon dioxide into chemical energy and organic compounds. It is of great scientific and technological significance to understand the mechanism of photosynthesis and optimize the efficiency and stability of photosynthesis. During the agriculture-mode cultivation process, plants and algae are required to withstand combined high light and high temperature (HLHT) stress, which disturbs photosynthesis activities and biomass production. Improving the efficiency and stability of photosynthesis has long been an important direction for plant breeding and crop improvement. Cyanobacteria are important model organisms for exploring and engineering photosynthetic mechanisms. The improvement of HLHT tolerance of cyanobacteria and the analysis of its functional mechanism have guidance and demonstration value for the optimization of other photosynthetic biological systems. However, there are many targets and complex mechanisms of damage caused by HLHT stress, so the traditional metabolic engineering and evolutionary engineering methods are difficult to achieve good results. Recently, the Microbial Manufacturing Engineering Center led by Prof. LV Xuefeng from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) has developed a hypermutation system in cyanobacteria, which eliminated the restrictions of cyanobacterial evolution through genetic and environmental co-disturbance, increasing replication mutation rate and adaptive evolution rate and the obtain of evolved strains with significantly improved tolerance to HLHT stress. What’s more, the key targets and functional mechanisms affecting the HLHT tolerance of cyanobacteria were revealed. The study was published online in Nature Communications on March 4, 2023. Fig. 1. Synergistic effects of inefficient replication fidelity mechanism and environmental stresses on genome replication mutation rates in Synechococcus cells. The rational metabolic engineering strategy is difficult to effectively improve the cell tolerance to HLHT stress because the damage mechanism of cyanobacteria caused by HLHT stress has not been clearly understood, while evolutionary engineering is a more effective strategy. However, due to the highly accurate genome replication machinery and the limited mutation rates, the classical adaptive laboratory evolution requires long-term continuous passage to accumulate genetic diversity, resulting in low efficiency, laborious and time-consuming. The research team systematically identified the key genes affecting its genome replication fidelity in Synechococcus elongatus PCC 7942 (Synechococcus), and increase the genome mutation rates of Synechococcus cells by over 100-fold through the integration strategy of fidelity element knockout and mutagenic element expression. On this basis, the synergistic effect of environmental conditions and genetic fidelity machinery on genome replication mutation rates was uncovered. The study shows that a hypermutation state can be triggered in strains carrying deficient fidelity machinery with elevated temperatures and light intensities of Synechococcus cultivation, and the cellular genome mutation rates can be increased by more than 1000-fold. Thus, a genome mutation rate regulatory mechanism was proposed for Synechococcus (Fig. 1), and the HLHT tolerance of Synechococcus was improved using the engineered hypermutation system. Fig. 2. Improving HLHT tolerance of Synechococcus using the engineered hypermutation system Utilizing the engineered hypermutation system, the HLHT tolerance of Synechococcus was improved within two weeks, which had great advantages over the adaptive laboratory evolution and the conventional chemical mutagenesis. Under the conditions of high temperature (45°C) or high light (500 μmol photons/m2/s) which WT couldn’t survive, the evolved strain showed good adaptability and rapid growth ability (Fig. 2). Fig. 3. NC2 mutations up-regulated the shikimate kinase expression to enhance HLHT tolerance in Synechococcus To explore the HLHT tolerance mechanism of Synechococcus obtained in the engineered hypermutable process, 23 tolerant strains were selected for genome sequencing, and functional mutations and mechanisms that determine the evolved traits were identified using forward genetic analysis and reverse genetic confirmation. It was found that NC2 (upstream non-coding sequence of synpcc7942_0894) and 0336 (AtpA-C252Y) mutations are the essential factors driving the adaptation process of Synechococcus cells under HLHT stress. Among them, the mechanism that NC2 mutations up-regulated the shikimate kinase expression to enhance HLHT tolerance in Synechococcus is reported for the first time. the homolog of synpcc7942_0894 (sll1669) was overexpressed in Synechocystis. What’s more, recombinant Synechocystis. sp. PCC 6803 strain overexpressing the sll1669 gene (shikimate kinase coding gene) showed optimized adaptation to increased light intensities at 30°C and 38°C, indicating that the overexpression of shikimate kinase might be a universal strategy for adapting cyanobacterial photosynthesis to light and temperature stress (Fig. 3). Fig. 4. NC2 mutation remodeled the photosynthetic metabolism in Synechococcus The team further revealed the mechanism that NC2 mutations up-regulated the shikimate kinase expression to enhance HLHT tolerance. Combined with the analysis of the transcriptome, proteome, and photosynthetic physiological parameters, it was found that NC2-1 mutation caused significant changes in the photosynthetic and carbon sequestration system, resulting in reduced light energy overabsorption, enhanced cell cycle electron flow and oxidative phosphorylation activity, and enhanced glycogen and protein synthesis, which ensures an efficient and stable process of photosynthetic carbon sequestration (Fig. 4). In this study, a new type of cyanobacterial hypermutation system was developed and was proved effective through the improvement of HLHT tolerance of Synechococcus, which provides a reliable tool for the optimization of complex photosynthetic physiological phenotype. What’s more, the mechanism that NC2 mutations up-regulated the shikimate kinase expression to enhance HLHT tolerance found in this study enriched the understanding of photosynthetic physiology and metabolism and provided new inspiration for the artificial design of cyanobacteria chassis with high photosynthetic efficiency in the future. Prof. LV Xuefeng and Prof. LUAN Guodong of the Microbial Manufacturing Engineering Center are the co-correspondent authors of the paper, and the doctoral student Sun Huili and Prof. LUAN Guodong are the co-first authors of the paper. This study is supported by the National Key Research and Development Program of China (Grant number 2021YFA0909700, to X.L. and G.L.), the Youth Innovation Promotion Association CAS (to G.L.), the National Natural Science Foundation of China (Grant number 32070084 to G.L., 32270103 to G.L., 32271484 to X.L.), the DNL Cooperation Fund, CAS (DNL202014, to G.L.), and the Shandong Taishan Scholarship (to X.L. and G.L.). Image by SUN Huili and LUAN guodong Text by SUN Huili and LUAN guodong