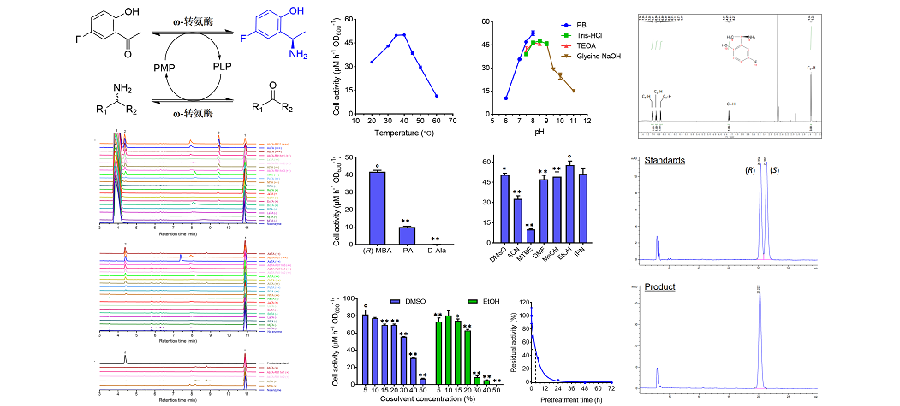
Chiral amines are important building blocks of many drug molecules. The asymmetric biosynthesis of chiral amines by enzymes is one of the most promising methods. Recently, researchers of MME and Sphinx Scientific Laboratory (Tianjin) developed an efficient method for the biosynthesis of the anti-tumor drug intermediate (R)-2-(1-aminoethyl)-4-fluorophenol using ω-transaminase. TRK (tropomyosin receptor kinases) abnormal expression in human tissues may cause the tumors and other diseases. As an important intermediate of TRK inhibitor-type anti-tumor drugs, the efficient synthesis of (R)-2-(1-aminoethyl)-4-fluorophenol is attracting increasing attention. Using a highly efficient ω-transaminase from Arthrobacter sp., the authors developed and optimized a method for (R)-2-(1-aminoethyl)-4-fluorophenol biosynthesis based on the whole-cell catalysis, and further established a complete process for product isolation and purification with high recovery rate and (R)-enantiopurity. The results of this study were recently published in the journal “Enzyme and Microbial Technology”. (Image and text by Guan Zhou & Quan Luo) https://doi.org/10.1016/j.enzmictec.2024.110406 Quan Luo#, Guan Zhou#, Zhongxia Li, Jiangpeng Dong, Hang Zhao, Huifang Xu*, Xuefeng Lu*. ω-transaminase-catalyzed synthesis of (R)-2-(1-aminoethyl)-4-fluorophenol, a chiral intermediate of novel anti-tumor drugs. Enzyme and Microbial Technology. 2024(175):110406.